James Watson and Francis Crick...
Discovery of the DNA structure
![]()
"We
wish to suggest a structure for the salt of
deoxyribose
nucleic acid (D.N.A.). This structure has novel
features
which are of considerable biological interest."
James Watson and Francis Crick...
Discovery of the DNA structure
![]()
"We
wish to suggest a structure for the salt of
deoxyribose
nucleic acid (D.N.A.). This structure has novel
features
which are of considerable biological interest."
Chapter 1. History
Unfolding
Chapter 2. Rosalind
Franklin and X- Ray Crystallography
Chapter 3. Building
the Model
Chapter 4.
Chargaff's 1:1 Rule
Chapter 5.
Tautomeric Forms
Chapter 6. Final
Structure


James D. Watson and Francis H. C. Crick shared the 1962 Nobel Prize
in Physiology or Medicine with Maurice H. F. Wilkins.
James Watson (1928-present) is an American from Chicago, Illinois. He was an extremely bright child, and entered the University ofChicago at age 15 to study Zoology. He graduated with a B.Sc. in 1947 and was accepted to Indiana University in Bloomington for graduate studies. There he studied bacteriophages under Salvador E. Luria ( 1912-1991) and received his Ph.D. in Zoology in 1950. He then spent a year in Copenhagen, Denmark as a postdoctoral researcher working on viruses. During this period he went to a conference in Naples, Italy where saw X-ray diffraction picture of DNA by Maurice Wilkins. After this pivotal incident he soon arranged to move to the Cavendish Laboratory of Cambridge University, England where he hoped to study DNA.
Francis Crick (1916-present) was born in Northampton, England and
attended the University College in London. He received a B.Sc. in Physics in 1937 and began work on his Ph.D., but was interrupted when World War II broke out. During the war he worked as scientist for the British Admiralty developing land mines. He left the Admiralty in 1947 to study biology, joined the Cavendish Laboratory in 1949 and soon began work on his Ph.D studying proteins by X-ray diffraction.
It was at the Cavendish Laboratory that Watson and Crick met.The lab was run by Sir William Lawrence Bragg (1890-1971), and also included Max F. Perutz (1914-present) and John C. Kendrew (1917-1997). Watson and Crick soon realized their common interest in the nature of the “genetic material”. Despite Avery, Macleod and McCarty’s transformation experiments, there was still a debate among scientists as to whether proteins or DNA were the genetic material. Even without knowing the identity of the molecule, it's general characteristics were established and scientists speculated on what the nature of the mystery molecule was. Watson and Crick were among those who thought nucleic acids were the key to hereditary transfer. With the Hershey and Chase experiment of 1952 they, and others, became more sure of this hunch. Watson and Crick wanted to determine the structure of DNA and hoped this would lead to a deeper understanding of how life is propagated
Linus C. Pauling (1901-1994) was one of the most important scientists of the 20th century. He did a great deal of important work on chemical bonds and protein structures, and his chemistry text (The Nature of theChemical Bond, 1939) was relied upon heavily by Watson and Crick for reference. Some of Pauling's most important work utilized X-ray crystallography to aid the structural determination of biological molecules. He recieved the Nobel Prize for Chemistry in 1954, and the Nobel Peace Prize in 1963. He also pioneered model building method of structural determination used by Watson and Crick, and by which he had elucidated the structure of the alpha helix. Plausable 3-dimensional models could be built from knowledge of chemical bonding and bond distances to fit experimental data.
Watson and Crick used this method to elucidate the structure of DNA. Without any experimentation of their own, they brought together other peoples expertise and data to build their model.
Principle contributions:
1) X-ray crystallography pictures and measurements from Rosalind Franklin
2) DNA base proportions from Erwin Chargaff
Many others also contributed greatly to Watson and Crick's famous discovery.
Rosalind Franklin and X-Ray Crystallography

Rosalind E. Franklin (1920-1958) was a colleague of Maurice Wilkins (1916-present) at King’s College, London at the same time Watson and Crick were working on DNA structure at Cambridge. She was trained as a chemist and had extensive experience with X-ray crystallography, but absolutely no experience with biology.
X-ray crystallography was pioneered by Sir William Henry Bragg and his son, Sir William Lawrence Bragg before World War I. The technique uses X-rays to obtain information about the positions of atoms in a chemical structure. It is based on the wave properties of X-rays and the ability of regular or symmetrical structures to crystallize. When a beam of X-rays is incident on a crystalline structure (i.e. one with an ordered arrangement of molecules), the X-rays are diffracted and will produce a pattern which can be recorded photographically. The resulting diffraction pattern can be complex to interpert, but can give information about the crystals atomic structure.
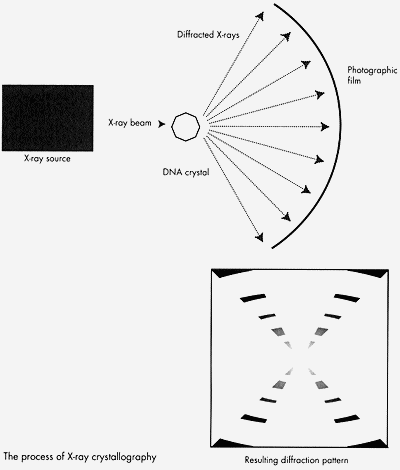
X-ray crystallography was originally used to look at the structures of simple organic minerals, but was progressively applied to more and more complex molecules. It aided in determining the structures of the alpha helix, the beta sheet, hemoglobin, and DNA.
In 1952, Franklin took the best DNA X-ray diffraction pictures thus far. W. T. Astbury, Wilkins, and others had taken DNA diffraction pictures before, but none had turned out as clear or precise. Franklin also distinguished between two types of DNA crystals, the "dry" and "wet" forms, later known as the A-DNA and B-DNA forms, respectively. The DNA crystals Astbury used actually contained a mixture of the two forms, complicating his diffraction pictures and calculations.Franklin's 1952 X-ray crystallography picture of B-DNA.
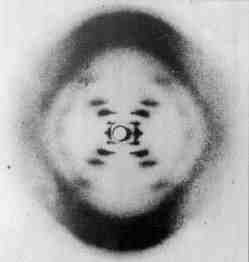
Watson was shown this picture by Wilkins in early 1953.
From the picture it was possible to calculate:
1) the distance between bases (3.4A)
2) the length of the period (34A)
3) the rise of the helix (36 degrees)

Lead sentence: "We wish to suggest a structure for the salt of deoxyribose nucleic acid (D.N.A.)."
Therefore, knowing that DNA existed and contained four bases, a ribose sugar and phosphate. Inspired by Pauling's successful attempts at building 3-D models of proteins, Crick and Watson believed this to be the correct way to proceed.
The first attempt at building a 3-D structure was an embarassing failure. This failure was blamed on Watson's faulty report of the data presented at a seminar given by Rosalind Franklin in November of 1951.
This attempt was followed by a retreat into thier repective "other" studies.... a delay lasting nearly 18 months. No models were built during this time, and Watson and Crick followed different trains of thought during this time...
Watson was considering the possible role of divalent ions in holding the chains in DNA together and had bacteriophage DNA tested in the flame photometer for the presence of small quantities of magnesium.
John Griffith, the mathematician nephew of Fred Griffith, calculated the attractive forces between 'like' bases. Crick's idea was that since the bases were flat, perhaps they coud be stacked on top of one another, and attracted that way. Griffith informed him that adenine attracted thymine and guanine attracts cytosine and Crick immediately saw the biological interpretation of this:
A goes to make B and B goes to make A
Preferential attraction between adenine and thymine and between guanine and cytosine woud give rise to .
Around this same time period, others beside Watson and Crick were attempting to solve the structure. In 1949, Furberg’s best proposed structure, a single chain in helical form with the bases sticking out flat and parallel to each other, retained Astbury’s correct distances, but was insufficiently dense. There was too little in it. Key information was unknown to Watson and Crick, although it had been published several years earlier.

Before Chargaff's chromatography work on nucleic acids, chemists seem undisturbed that thier base analyses failed to approach the requirements for the tetranucleotide theory, claiming that their methods were too crude.
Chargaff used qualitative paper chromatography developed by Martin and Synge (1944) for the amino acids. The technique involved nucleic acid, filter paper, and a Beckman photometer. Purines and pyrimidines were analyzed by conversion to thier mercury salt, marking the analogue and eluting that. Despite the clumsiness of the technique, it worked.
Chargaff was anxious
to disprove the tetranucleotide theory, and to also establish a parallel between
taxonomic relationship and DNA composition; that DNA was characteristic of the
species, but not of the tissues. What he also got was a remarkably close
approach to equality between the now well known pairs of bases, whether he
wanted it or not:
"A comparison of the molar proportions reveals certain
striking,
but perhaps meaningless,
regularities"
(Vischer, Zamenhof and Chargaff. 1949. Microbial nucleic acids:
The
Desoxypentose Nucleic acids of avian tubercle bacilli and yeast.
J. biol.
Chem. 177: 429 - 438)
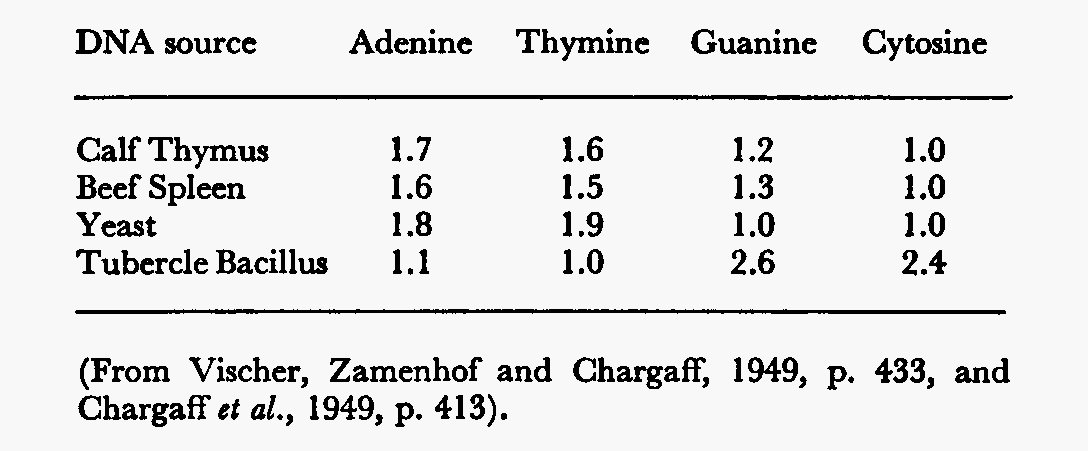
This data insinuated that the structure of the DNA was specific and conserved in each organism. He also found, more importantly, that in all animals:
These quantitative relationships are important, not only in establishing the three-dimensional structure of DNA, but also in providing clues on how genetic information is encoded in DNA and passed on from one generation to the next. When Crick compared the bases that Griffiths had told him with Chargraff's data, he realized that complementary base pairing could be the cause of the 1:1 rule.
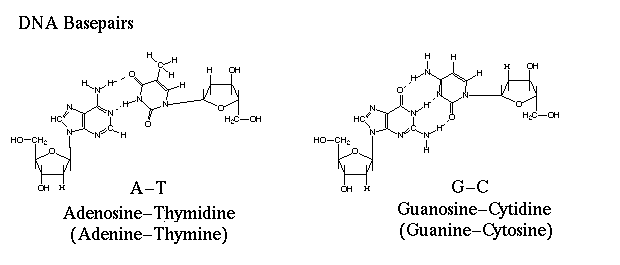
What stalled Watson and Crick was the thought that hydrogen bonding was too unstable to be responsible for replication. Crick was also assuming that both tautameric forms of the bases existed in the same DNA molecule, and that the proton could shift from one position to another, thus altering the sites for hydrogen bond formation. If the sites varied in the same base, you could not get the consistency necessary for replication.
Guanine and thymine
can have alternate molecular structures based on different locations of a
particular hydrogen atom. A keto structure occurs when the hydrogen atom bonds
to a nitrogen atom within the ring. An enol structure occurs when the hydrogen
atom bonds to an nearby oxygen atom that sticks out from the ring. Both guanine
and thymine can switch easily from one tautomer to another. The change in shape
affects the three-dimensional shape of the molecule. By using Keto instead of
enol forms, adenine thymine pair and a guanine-cytosine pair could be
formed.
The final model proposed by Watson and Crick was 'pushed' by the publication of a potential model by the Pauling-Cory group. While examining a pre-press copy of the paper, a fundamental mistake was noted. Although Pauling was aware of Avery, Macleod and McCarty’s work, he was not convinced DNA was the genetic material and was convinced that proteins were most likely the prospects because of their variability (20 amino acids). Despite this, he decided to work on the structure of DNA anyway.
Pauling proposed a triple-helical model for the structure of DNA during the early months of 1953. The model had three intertwined chains held together by hydrogen bonds. The sugar phosphate backbone of each chain was oriented towards the axis and the bases pointed out.
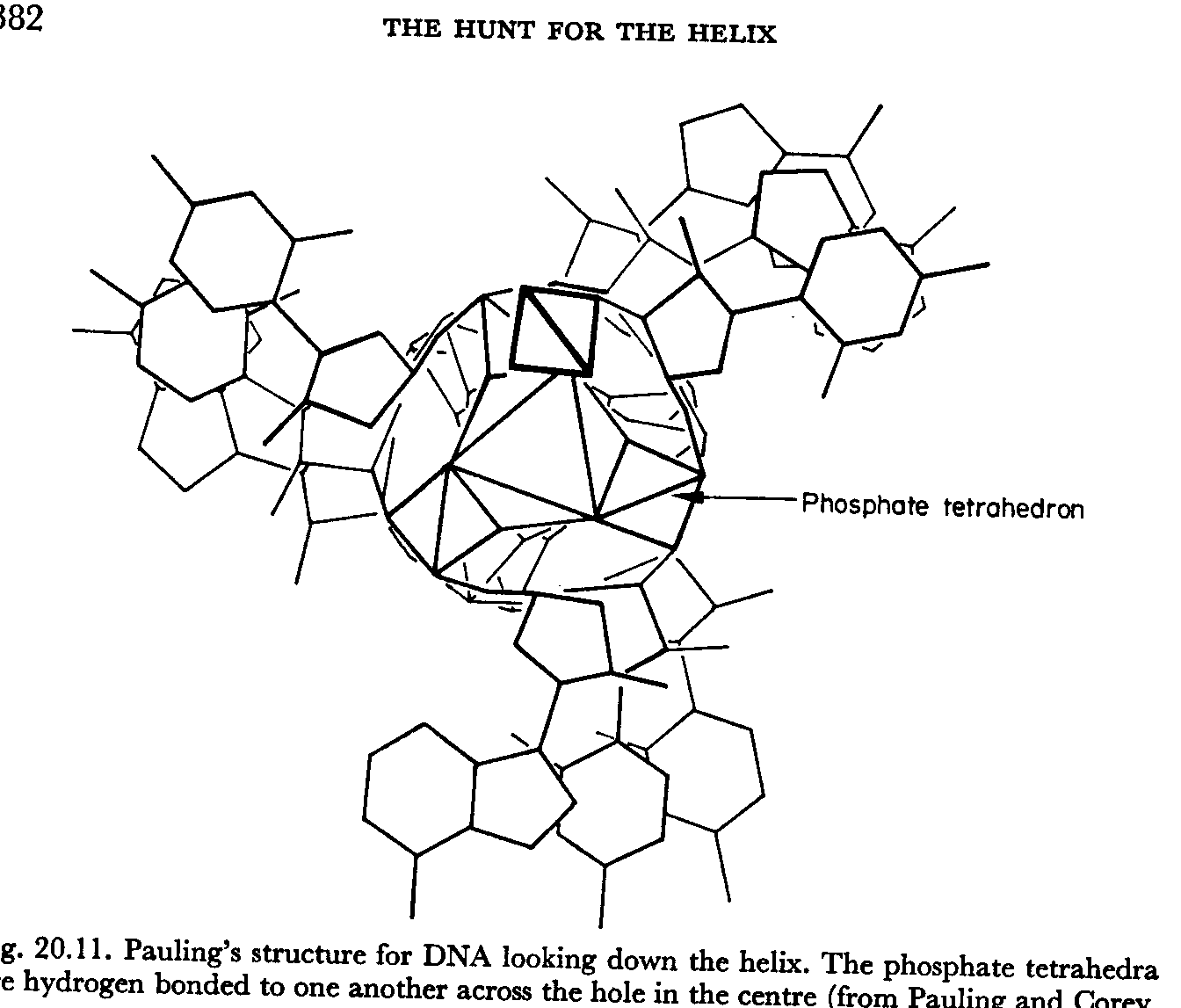
Pauling had
forgotten the negative charges of the oxygen atoms in each phosphate group.
Facing towards the middle and stacked on top of one another, these charges would
repel each other, making it impossible for the molecule to hold together.
Knowing that this mistake would not go unnoticed for long, Watson and Crick
redoubled thier efforts and developed thier own model within weeks of recieving
Pauling's paper. This model was one of the first points to be addressed in the
April 1953 paper by Watson and Crick:
"Their model consists of three intertwined chains, with the phosphates near the fibre axis, and the bases on the outside."
The paragon of
elegance, this paper is renowned for its simplicity, clarity, durability and
understatement. The four key characteristics of this model of DNA endure: DNA is
, ,
, and the double strands are in a
. And the authors, James Watson and Francis Crick,
were 25 and 35 years old, respectively, when the paper was published.
"This structure has two helical chains each
coiled round
the same axis (see
diagram)."
Characteristics 1 and 2: DNA is double-stranded in a double-helix.
"We have made the usual chemical assumptions, namely, that each chain consists of phosphate diester groups joining ß-D-deoxyribofuranose residues with 3',5' linkages."
"Both chains follow right- handed helices, but owing to the dyad the sequences of the atoms in the two chains run in opposite directions."
"The novel feature of the structure is the manner in which the two chains are held together by the purine and pyrimidine bases.... if only specific pairs of bases can be formed, it follows that if the sequence of bases on one chain is given, then the sequence on the other chain is automatically determined."
"We have assumed an angle of 36 degrees between adjacent
residues in the same chain, so that the structure repeats after 10
residues on each chain, that is, after 34 A. The distance of a phosphorus
atom from the fibre axis is 10 A."
"As the phosphates are on the outside, cations have easy access to them."
The phosphates are negatively charged, and attract cations (net
positive charge). The phosphates, being charged, are also hydrophilic.
"It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material."
For their outstanding work in discovering the double helical structure of DNA, Watson and Crick shared the 1962 Nobel Prize for Physiology and Medicine with Maurice Wilkins. Sadly, Rosalind Franklin, whose work greatly contributed to this key discovery, died before this date, and the rules do not allow a Nobel Prize to be awarded posthumously.
Questions or comments about this site?
contact: Anne-Marie
Gale
Genetics, Evolution, and Molecular Systematics Laboratory
SN-3007, (709)
737-4713
Department of Biology
Memorial University of Newfoundland
or Jay Penny
For References